Developed by Paul Anastas and Julie Zimmerman*, these engineering principles outline what would make a greener chemical process or product. See also the Sandestin principles of green engineering.
Click on the tabs to reveal articles about each principle. These articles were originally developed for The Nexus Blog.
-
Designers need to strive to ensure that all materials and energy inputs and outputs are as inherently nonhazardous as possible.
Contributed by Dr. David Constable, Director, ACS Green Chemistry Institute®
All chemicals have properties that help us to characterize and differentiate them from each other. Chemists and chemical engineers are most familiar with properties like boiling point, melting points, freezing points, vapor pressure, water solubility, and so forth. Chemical engineers are also more familiar with properties like flammability, explosivity, compressibility, viscosity, and other properties that affect heat and mass transfer, etc. Most chemists and chemical engineers are less familiar with properties related to toxicity to environmental organisms and humans, and this is one of the things this principle has in view.
The second thing this principle has in view is a systems perspective; i.e., the ability to do mass and energy balances around a unit operation, a chemical process, a facility, or an even larger, more comprehensive and complex system like an industrial park or petrochemical complex. Fundamental to a mass and energy balance is the ability to map inputs and outputs to and from a system or around any boundary you may wish to define.
A third thing this principle assumes is the importance of design and the key role designers. Bill McDonough in his book “Cradle to Cradle” says that design is a signal of intent. I have always felt that this is a great summation of the importance design; it’s absolutely critical to have the best intention. This principle is asking designers of manufacturing processes or products to actively intend that the materials and energy used to make a product have the lowest adverse impact possible.
In the case of materials, designers need to select chemicals or materials made from or with chemicals whose properties will not cause harm to the environment or to people throughout their life cycle. In the case of energy, most designers capitulate to what is readily available – electricity and steam – both of which owe their production primarily to fossil fuels. However, not all energy is created equal in terms of its toxicity profile and its overall efficiency (conversion through transmission through use), even if one is constrained to fossil fuels. Moreover, with the right choice of chemicals and materials, a designer can control how much energy is required and the form of that energy; e.g., heating, cooling, light, microwave, pressure, etc. So please don’t capitulate. Energy matters in terms of putting toxics into the environment as much as, or is some cases, more than the choice of chemicals.
Green engineering demands—even in just this first principle—that you pay attention to a larger set of specifications or design constraints than you otherwise might. If all you intend to do is make something new and bring it to market as quickly as possible, this can at first seem overwhelming. But this is the task at hand if we want to live in sustainable world.
-
It is better to prevent waste than to treat or clean up waste after it is formed.
Contributed by Dr. Martin Abraham, Dean, College of Science, Technology, Engineering, and Mathematics, Youngstown State University
One of the central tenets of all green technologies is to make only the amount that is needed for the process at hand. From a business perspective, this makes absolute sense. If you purchase a quantity of a chemical, bit only use half of what was purchased, you will need to properly dispose of the remainder. You pay for the initial purchase and then pay the disposal costs, paying twice for something you didn’t need in the first place. A related concept can be found in health care, in which we suggest vaccination to prevent disease. Better to get your flu shot in late fall than to suffer the agony of having the flu in the winter.
One can easily extend this to the concept of preventing waste in chemical reactions. For a chemical reaction, the important question to ask is not only how much of the desired product can be formed, but also, how much of the undesired product is formed (and how much will need to be thrown away). We can characterize this parameter as the selectivity, or the ratio of one product relative to another. In a general sense, this can be written as:
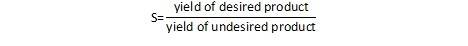
An alternate definition provides selectivity as the ratio of the amount of the desired product relative to the total conversion.
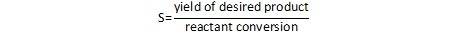
I often use the reaction of ethylene to produce ethylene oxide as an example. This is a standard oxidation reaction performed in the vapor phase over a silver catalyst. Since this is an oxidation reaction, the desired ethylene oxide product can also be converted to CO2. In this process, we pay for the ethylene, we pay for the energy required to run the reactor, and we get no economic benefit from the CO2 that is produced. From a purely economic standpoint, the reaction is best performed in a way to maximize the selectivity to ethylene oxide, keeping the amount of CO2 formed to a minimum. CO2 is also a greenhouse gas that has been implicated in climate change, so it’s transmission to the atmosphere is clearly undesirable.
Fortunately, there are many tools available to engineers and chemists to reduce the amount of waste that is formed in a process. For a reacting system, adjusting the temperature will cause one reaction to be accelerated more so than another, and thus the selectivity to the desired produce may be increased. If selectivity to the desired product is enhanced at lower temperatures, then the reaction may not proceed sufficiently fast in order to be commercially interesting, so the addition of a catalyst may be needed. Also, it is possible that manipulating the concentration of one or more of the reactants may be effective in modifying the relative rates of the reaction. And finally, the engineer might design a process in which the reaction is run to low conversion, a separation is achieved to recover the product, and the unused reactant is recycled back to the entrance of the reactor, allowing higher overall conversion to be obtained. If one includes the cost of waste disposal into the process optimization, then manipulating the process parameters allows will the engineer to adjust the performance of the system to achieve the least costly overall solution, and the one that produces the lowest amount of waste.
-
Separation and purification operations should be designed to minimize energy consumption and materials use.
Contributed by Dr. Matthew J. Realff, Professor and David Wang Sr. Fellow, School of Chemical and Biomolecular Engineering, Georgia Tech
Industrial separation processes are very energy intensive and in most cases have not approached the thermodynamic limits of minimum work of separation [1]. Historically, for liquid and condensable gas separation, multistage distillation has been the workhorse process, based on boiling point differences between the components to be separated. The energy consumption of oil refineries involved distilling crude oil into its various fractions and subsequent separation of thermally or catalytically cracked components in these various fraction. Moreover, many bulk organic chemicals involve distillation in their production, which adds significantly to their production CO2 footprints. Thus, avoiding distillation, making distillation more efficient, and searching for alternatives to distillation are very important aspects of implementing the third principle of green engineering.
How can distillation be avoided? One approach is to integrate the process reactions with the separations to avoid the generation of mixtures that have to be separated. For example, methyl acetate is produced along with water from methanol and acetic acid. This reaction is equilibrium limited and results in a mixture that has several binary azeotropes between the components. The traditional process involved the use of eight distillation columns, one liquid extraction and a decanter[2]. This complex series of units was replaced by a single unit that integrated the reaction into the separation process[3]. The key insight is that if one of the products, in this case methyl acetate, leaves in the vapor phase, and the other, water, in the liquid phase, the equilibrium conversion is avoided and the feeds can be completely converted to products. This results in a system that is 1/5th the capital and energy cost of the traditional process[2].
How can distillation be made more efficient? The energy consumption in distillation occurs in the reboiler where heat is used to create the vapor stream that travels up through the column and whose composition is enriched in the more volatile component by stripping it from the downward flowing liquid. This vapor is then condensed to a liquid product and a fraction returned to the column to contact the rising vapor from which the less volatile component is absorbed as the liquid travels down the column. This complex process creates the desirable composition gradient in the column, with the higher boiling component more concentrated at the reboiler, and the lower boiling component at the condenser. Typically, the heat of condensation is released is at a lower temperature than the heat of vaporization is absorbed, but what if the temperature of the vapor could be increase before it were condensed? In fact, vapor compression achieves precisely this outcome– where the work of compression is used to raise the vapor temperature and then reuse the heat matched to the reboiler. This improves the energy efficiency of the distillation column and can lead to separation processes for dilute products that would not be energy efficient otherwise[4].
What about alternative separation techniques to replace distillation? Distillation does not take advantage of the very specific differences in molecular size, or other physicochemical properties such as affinity for specific solvents or solid adsorbents and so there are more selective separation techniques that would potentially use less energy than distillation. Unfortunately, many of these techniques fail to scale up as effectively as distillation to the large flow rates required for industrial chemical production at world scale plants. Moreover, distillation enjoys the advantage of inertia – with a large installed industrial base and many years of experience in operation. These factors have hampered attempts to replace distillation with other more energy efficient technologies.
One technology that has broken the hold of distillation in a large scale application is reverse osmosis membrane separation for water desalination. Reverse osmosis uses mechanical pressure to overcome the osmotic pressure exerted by the salt solution and thereby push the water through a selective skin. The theoretical energy to de-mix water and salt is approximately 1 kWh/m3 of water, the current best membrane technologies have a real energy cost of 4.0 kWh/m3[5] and thermal “distillation” type technologies use on the order of 50 kWh/m3[6] . In this case the size difference between a hydrated salt ion and a single water molecule is a factor of 3 and the membrane matrix allow the water to cross but retain the salt ions. Membranes for this application are manufactured efficiently at very large scale and a large surface area can be packed into a relatively small volume, both of which factors enable the technology to not only be competitive from an energy perspective but also from an overall cost perspective.
To implement the third principle of green engineering, more applications of energy efficient, but often more capital intensive technologies, will have to be developed. A key enabler will be the combination of highly selective materials that can “grab” or “pass” certain molecules from those that are closely related in size or other properties, with manufacturing technologies, such as hollow fiber membranes, to lead to scalable energy efficient separation methods. Reverse osmosis membrane desalination is a leading example of such an approach and other applications are under active development.[7]
[1] E. L. Cussler, B. K. Dutta, Aiche Journal 2012, 58, 3825-3831.
[2] J. J. Siirola, in Advances in Chemical Engineering, Vol. 23 (Ed.: G. Stephanopoulos), Academic Press, London, 1996, pp. 1-62.
[3] V. H. Agreda, L. R. Partin, W. H. Heise, Chemical Engineering Progress 1990, 86, 40-46.
[4] D. Luo, Z. Hu, D. G. Choi, V. M. Thomas, M. J. Realff, R. R. Chance, Environ. Sci. Technol. 2010, 44, 8670-8677.
[5] S. A. Avlonitis, K. Kouroumbas, N. Vlachakis, Desalination 2003, 157, 151-158.
[6] N. M. Wade, Desalination 2001, 136, 3-12.
[7] R. P. Lively, R. R. Chance, B. T. Kelley, H. W. Deckman, J. H. Drese, C. W. Jones, W. J. Koros, Industrial & Engineering Chemistry Research 2009, 48, 7314-7324.
-
Products, processes and systems should be designed to maximize mass, energy, space, and time efficiency.
Contributed by Dr. Michael A. Gonzalez, Senior Chemist, US Environmental Protection Agency, Office of Research and Development, Cincinnati, Ohio
As one reads the twelve principles of Green Engineering, there is one message that stands out and becomes ever increasingly more evident with each and every principle. And, that message is simplicity! It is simplicity that will allow us, as a society, to become more sustainable.
Although I am academically educated and trained as an Inorganic (Catalyst) Chemist, I have had the fantastic opportunity to interact and collaborate with Chemical Engineers during my entire tenure at the US EPA. These daily interactions have been extremely educational to myself and have focused my chemical research to think and design for development and application “beyond the bench”.
So what is meant by “beyond the bench”? Organic chemists are taught to focus on developing chemical reaction schemes that arrive at the desired structure, with all necessary functional groups in the correct positions. But, this has largely been done so with only the correct final molecular structure as the goal. Not, with any consideration regarding the implications associated with the complexity of the reaction, and material, energy and production requirements that will be needed to take this chemical reaction to the next larger scale. This is where “beyond the bench” thinking is prudent and required. As chemists, we can utilize our chemical knowledge, as molecular architects, to influence and accelerate the development of sustainable chemical design, synthesis and production. By making simple changes and decisions on a chemical synthesis route at the bench scale, not only are green and efficient reactions able to be designed. But, these changes have the potential for significant beneficial impacts when taken to the next higher scale of production.
Green Engineering Principle #4 focuses on maximizing efficiency. This is achieved by informing scientists and engineers to create designs that maximize efficiency in multiple areas such as mass, energy, space (i.e. real estate) and time. This is a simple and logical path that should be taken and the benefits gained can be quite significant. However, rather than focusing on these areas individually, by integrating these areas the benefits gained can be further increased. This is due to the high interdependence of one area on another.
In the area of mass efficiency, it is evident that all reactions should be designed to utilize as much of the reactants as possible. Reactions should be designed to catalytic or have stoichiometries as close to what is required for the reaction. As well as have high conversions and be selective to the desired products, with minimal by-product formation.
In the area of energy efficiency, it is desirable to stay as near to room temperature and pressure as possible. The need to heat and cool over large ranges requires substantial quantities of energy and can also be quite inefficient. Especially, if the chemical synthesis route requires a number of heating and cooling cycles. With this in mind chemists need to be cognizant of the subsequent steps in a synthesis sequence and design the route to utilize the heating (or cooling) that has already been committed to the reaction in a preceding step. Additionally, if you can minimize the mass of materials being moved within a chemical process, you are also contributing to an energy savings. This is the result of not needing to pump, stir or temperature control a larger than necessary mass of materials. Designing reactions that produce product streams that are as pure as possible can also experience the same energy gains. Thus, reducing the need for separation steps and recycle loops and the energy that drives these operations.
In the area of space, or in this context real estate, it is prudent to design reactions (and their subsequent processes) to be as small as possible. Not only does this contribute to a smaller physical footprint, it can also lead to a reduced environmental footprint. By having reaction volumes that are smaller, the heating and cooling load demands become reduced. The increased need for expensive materials to construct larger reaction vessels or processing equipment is reduced. And, by processing smaller volumes there is an increase in the safety of operations being performed. You can also imagine benefits in reduced risk of operations, economic gains, and reduced insurance costs to name a few.
And finally, in the area of time. I remember back to my graduate schools days and still see a sign that hung in our laboratories. It read “Time is Money”. As a young adult I had an idea what it meant. As a chemist, it took me a few years to really understand what it truly meant. In this context of time efficiency, the longer a chemical reaction takes for completion, or at least what you determined was the point of completion, the more money that was being consumed on materials, energy and operations. In addition, the longer the reaction runs, it is consuming valuable reactor real estate preventing other reactions from being performed. Which in turn is costing you additional money. When we describe time efficiency, we are identifying opportunities to perform chemical reactions as quickly as possible, while still being as efficient as possible.
Hopefully, I have demonstrated how interdependent these areas of efficiency are with one another. As you put all these concepts together, the opportunities for increasing the sustainability of a chemical reaction or process is increased. And, at the heart of these opportunities lies chemists and chemical engineers, like you and me, who can make smart and simple changes at the onset which can lead to tremendous gains and benefits at the process level. This truly demonstrates that thinking holistically can lead to something beautiful.
So remember, think and design for “beyond the bench”.
-
Products, processes, and systems should be "output pulled" rather than "input pushed" through the use of energy and materials.
Contributed by Michael A. Matthews, Professor of Chemical Engineering, Associate Dean for Research and Graduate Education, College of Engineering and Computing, University of South Carolina. Fellow of the American Chemical Society.
There is a familiar saying, attributed to Abraham Maslow (1966), that, to a man with a hammer, everything looks like a nail. The chemical and energy industries historically have been driven by inexpensive and abundant raw materials (the hammer): natural gas for fuels and chemicals, and coal for power plants. Very recently (Kaskey, 2013), improvements in drilling technologies have led to an increase in supply and decrease in cost of natural gas, encouraging both the fuels and chemicals industries to expand utilization of this essential clean resource for both power and chemical products (the nails).
The importance of inexpensive raw materials to the chemical enterprise cannot be discounted. Material availability, combined with the economies of large-scale facilities, have brought enormous value to the quality of life. Generic feedstocks and large facilities, however, will inevitably generate some waste. Principle 5 suggests that the availability of hammers is not the only consideration; rather, one must consider the real and immediate need for items to be nailed. The need should pull the act of production, rather than the ease and cost of production driving the need.
Three-dimensional printing (3D) is a most recent example of this principle in action. A diversity of products have been produced, ranging from automotive parts to a bronchial trachea for an infant. In 3D printing, the desired product is produced with layer-by-layer deposition of the appropriate resin. Biomedical applications, such as artificial trachea, are an outstanding example of “output-pulled” manufacturing. The exact, unique dimensions for the individual patient are determined by a variety of medical imaging technologies. A digital model is then produced, and the model drives layer-by-layer printing of the product, with no wasted raw material.
Electrical power production is another area where Principle 5 is beginning to be implemented. Most power today is produced in centralized, large-scale plants, including hydroelectric facilities. Distributed generation is the use of small-scale converters to produce power as needed for a localized facility, such as a home or a single building. The generator is chosen to suit the availability of local resources, such as a solar cell array in sunny areas, or small wind turbines in windy areas. In the future, as the technology of biomass conversion increases, there is the prospect of building small power plants or even solid oxide fuel cells to convert biomass waste to electrical power, on-demand, to satisfy local demands. Where excess power can be produced locally, it can be fed back to the grid. Ideally, this will reduce the need for new, large-scale power plants.
Chemical engineering education emphasizes the economies of scale of large plants. However, there are many examples of small-scale, specialty production facilities in existence, and the examples above illustrate additional opportunities. Future education should include the realization that sometimes the output-pulled approach to design and manufacturing will be best. Moving to a greener chemical and materials manufacturing enterprise calls for deeper understanding and the ability to balance and evaluate all principles of green chemistry and engineering.
References:
Maslow, Abraham H. (1966). The Psychology of Science—A Reconnaissance. p.15.
Jack Kaskey, Business Week, Chemical Companies Rush to the U.S. Thanks to Cheap Natural Gas. July 25, 2013. Accessed at http://www.businessweek.com/articles/2013-07-25/chemical-companies-rush-to-the-u-dot-s-dot-thanks-to-cheap-natural-gas
-
Embedded entropy and complexity must be viewed as an investment when making design choices on recycle, reuse, or beneficial disposition.
Contributed by Dr. Michael A. Gonzalez, Senior Chemist, US Environmental Protection Agency, Office of Research and Development, Cincinnati, Ohio
The evolution of nature’s products, systems and life forms has taken billions of years and still is an evolving system. Embedded in this evolution is a series of networks and larger systems that offer a level of complexity that we are just beginning to understand and experience their interconnectedness. It is with this new understanding of tradeoffs, unintended consequences and the implications of our actions that has established the scientific areas of sustainability and resilience and the contributing fields of green chemistry and green engineering, to name a few.
As continued innovations and advancements are being made by applying the tenants of green chemistry and green engineering, linkages regarding the impacts of these novel developments are being demonstrated daily. It is in these crossovers where important improvements made in one discipline cannot be lost or minimized when transitioned into another discipline. This rationale needs to be followed when applied in both the forward and reverse directions. A simple example of the need for this thinking is demonstrated when improvements achieved on a bench-scale chemical reaction are no longer being applied at the process-level due to difficulties associated with the scale-up. Thus, the improvement is no longer relevant and there is no net gain, or even a loss, in the overall performance results. This concept can also be extended when needing to retain the embedded complexity between your beginning and ending states.
The complexity in a beginning state depends on factors such as the number of atoms and/or functional groups present in a molecule, the type, quality and quantity of energy within a system, and the economic value in a starting material or process. Historically, when discussing retaining the value of a starting material throughout a process, ‘value’ has generally been implied to mean only a material’s economic value. This concept is very easy to understand in practice because it would not appear sustainable to have a method/process that operates by taking a material of high economic value and transforming it into one with a lower value. This is the same as having a business model that operates at a loss. If you were to have a business that did this, you would not be in business very long. Unless you like losing money.
This concept of retaining complexity can also be applied to the end-of–life (EOL) considerations for products. We have seen tremendous efforts placed on the design of chemicals and products that have reduced or minimized impact to the environment and human health during the use-phase of their life cycle. This has resulted in significant strides in identifying and providing alternatives to more impactful chemicals and products in use. Recently, this concept has been further extended by considering the EOL phase and focusing on the design of products to breakdown naturally or be designed for reuse, recycle and repurposing.
It is generally understood products which require high-entropic substances, greater mass and number of materials, more energy and time for their construction and the use of exotic manufacture techniques are defined as complex. Current approaches for recycling these complex products comes at an economic, environmental and societal price. Additionally, the embedded complexity, either natural or man-made, in the product is reduced, sacrificed and results in a down-cycled product (one with reduced complexity). Additionally, the high-level of embedded complexity in these products make them less attractive for recycling and limits their second life to reuse only. This is problematic as eventually their usable life time will be reached and then society has to address its disposal, the resulting waste and the entrainment of high-complexity materials that we cannot easily access for additional uses.
Because of these concerns, attention is being directed towards designing products with characteristic that are favorable for recycle, reuse and repurposing. Such approaches include products made with construction techniques that allow for easy, fast and safe disassembly, use of materials that have minimal complexity and are abundant, modular structures that allow for upgrades of the technical components without the need to entirely replace the product, and utilization of the fewest number and least different types of materials. While this is only one approach for EOL considerations in product design, this approach clearly demonstrates the application of holistic thinking.
As we, scientists and engineers, move forward in our individual research areas, it is important we think holistically and be mindful of the upstream and downstream considerations of our discoveries. We also need to remember and realize that while a chemical or product may not appear complex, there has been a considerable amount of time, energy (solar and thermal) and materials utilized in its production, either by nature or man, and it is our responsibility to ensure we utilize these embedded qualities efficiently and effectively. These actions will allow our discoveries and designs to be readily available for their next use or purpose with minimal altering and loss of complexity.
-
Targeted durability, not immortality, should be a design goal.
-
Design for unnecessary capacity or capability (e.g., "one size fits all") solutions should be considered a design flaw.
-
Material diversity in multicomponent products should be minimized to promote disassembly and value retention.
-
Design of products, processes, and systems must include integration and interconnectivity with available energy and materials flows.
Contributed by Dr. Concepcíon Jiménez-González, Director, New Product Development Clear Skin, Stiefel Skin Health, a GSK company
When we spend years working on a new synthesis to produce the next big reaction or molecule, it is very easy to keep our focus in our little world of lab reactors, chromatographic columns, HPLC and all the other cherished equipment. Perhaps we get so familiar with one part of the process that we now know the most intricate details of the reactor and the reaction, and have our ideas on how to maximize its efficiency.
However, when transferred into a larger scale, a chemical process is a system of interrelated units, inputs, outputs, and recycle streams. Even though it may be easier to study separate units as we are getting to know their inner workings, chemical process is not really a set of separated parts, but a complete unit where the individual components are intimately interrelated – similar to our bodies, with overarching systemic functions in addition to the individual organs.
The principle of Integrating Material and Energy Flows reminds us to treat processes as an entire system, and use the inter-relationships of the parts to our advantage. Chemical Engineers would recognize this as the application of Process Integration. Process integration is best understood as a holistic, systematic framework to optimize the mass and energy required for a given process.
Of HEN and MEN
Mass and energy are of course intimately related in any production process, since for instance a chemical process can be described as the way by which we convert mass and energy into a more valuable product. In a chemical process, we start with raw materials (mass) and then require steam (energy) to carry out chemical and physical transformations. As a result, we typically will have several hot streams that need to be cooled and several cool streams that need to be heated. To achieve this, one could cool all the hot streams with say, cold water or refrigerants and then heat all the cold streams with stream; but by doing this the total energy requirements would be maximized. To minimize the amount of utilities that need to be used, and therefore minimize costs and environmental impacts, we use process integration – specifically energy integration.
Energy integration can be simply described as using hot streams to heat cold streams, and vice versa, before any additional utilities are used, with the result of reducing the overall use of utilities. The simpler example is a heat exchanger – but this could be very complex, depending on the system, and one can need an entire network of those heat exchangers in the plant for effective energy integration. These networks are known as Heat Exchange Networks – or HEN. There are many examples of successful heat integration in a wide variety of industries, such as refineries, petrochemical, chemical, food and drink, pulp and paper, and metallurgical. The net results of heat integration applied successfully are cost savings, increased throughput, and reductions in emissions and environmental impacts.
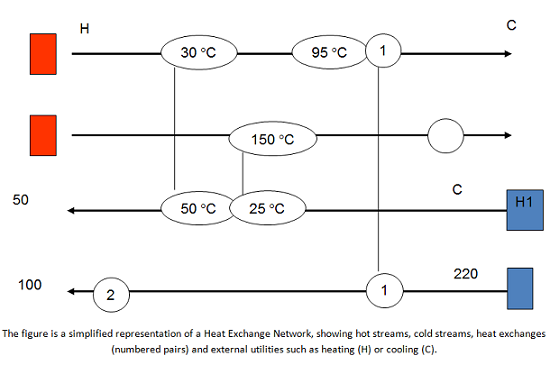
Mass Exchange Networks, or MEN, are the parallel concept for using process streams to integrate mass. MEN are very similar to HEN, but instead of exchanging energy using process streams, we exchange mass. For instance, very often we need to separate unreacted species from our desired product. We could use an additional material (say, a solvent) to remove the unreacted material and send it back to the reactor. However, that comes with additional cost and environmental impacts. By using MEN, we take advantage of lean streams (streams with low concentrations of a given material) to separate and recover mass from rich streams. The idea is to use as much of the lean streams to recover and potentially recycle the materials, before we have to use an external agent, thus reducing environmental and life cycle impacts in the process.
Of course, designing HEN and MEN is easier said than done – the mass and energy transfer is governed by thermodynamic and equilibrium laws, and thus will require your friendly neighborhood chemical engineers to do some calculations and graphs to produce the design– but that is where the fun begins.
If you want to get into some of the details on how to integrate mass and energy, the papers below may help.
- El-Halwagi, M. M. Process integration. In Process Systems Engineering, Vol. 7, Academic Press, San Diego, CA, 2006.
- Dunn, R. F., El-Halwagi, M. M. Process integration technology review: background and applications in the chemical process industry. J. Chem. Technol. Biotechnol., 2003, 78, 1011–1021.
- Smith, R. State of the art in process integration. Appl. Therm. Eng., 2000, 20, 1337–1345.
- Jimenez-Gonzalez and Constable, Green Chemistry and Engineering – A practical design approach. John Wiley and Sons, 2010.
-
Products, processes, and systems should be designed for performance in a commercial "afterlife."
Contributed by Dr. Concepcíon Jiménez-González, Director, New Product Development Clear Skin, Stiefel Skin Health, a GSK company
Do you own a computer? A mobile phone? A car? A TV set? How often do you get a new computer, gaming console, phone? Do you know what happens to the old ones when you are more concentrated in finding out how the features work in the new device?
Principle 11 helps us to think about those aspects as we are designing new processes, products and services. The idea is to make it easy for the user to do the right thing, and avoid unnecessary impacts after a product or process has reached the end of its usable life. If we do not take into consideration end-of-life aspects, we can have literally tones of materials either in landfills or being savaged in less than safe conditions.
To apply this principle in practice, McDonough and Baumgarteni propose creating technical nutrients for some materials in common use so that they may be used over and over again, being returned to their original state through low-impact processes. A great example of this may be found with DuPont’s Petretec process.ii DuPont has found a way to take any polyester and unzip the polymer to release the virgin monomer, which may then be reused to make “new” polyester products of many kinds. The process can be used to reclaim monomer from mixed-material streams containing polyester and is readily integrated into existing polyester manufacturing facilities. This diverts polyester from landfills or from waste to energy applications, and reduces the overall environmental impact.
Applying this principle to electronics design can help engineers create features that enable the recovery of materials for reuse into products of same or higher value – to either recycling or up-cycling them. Disassembly features allow for the quick sorting and removal of components and materials for servicing. For example, some of the strategies that Sun Microsystems incorporates in product design to enable ease of disassembly are:
- Product upgrades are planned intentionally to prevent the premature retirement of materials.
- Many components, such as boards, memory, and disk drives, can be added or replaced by the latest technology improvements.
- Once recovered, these components can be refurbished and sold as re-marketed equipment, or can be disassembled to separate valuable components for reuse elsewhere.
- Instead of using permanent methods such as ultrasonic welding or spray coatings to unite components, engineers can design shields with the minimum number of bonding points, or they can snap-fit materials so that metal shields and plastic housings are easy to separate.
- Embedded ISO 11469 identification codes for plastic type on plastic parts increase the chances of reuse and make it easier to sort materials that are in demand.
- Thin-walled plastic design conserves the amount of material needed while maintaining strength requirements and yields extra environmental benefits by reducing the amount of fuel needed to transport new, lighter products.
- Non-painted plastics make recycling and recovery easy.
You may want to take a look at this short video from Sun Microsystems' CTO Greg Papadopoulos describing the overall philosophy.Another example of this attention to recycle and reuse maybe found in how modern Xerox machines are developed and marketed. Xerox uses and reuses electronics, optics, and other components over and over in new products or products that are changed, upgraded, and expanded over time. Materials that cannot be reused are either recycled into other product streams or become raw materials for new parts.iii Another example is the automobile industry, where about 95% of the average vehicle is recycled in one form or another, thereby avoiding huge quantities of landfill waste.
Please think about the opportunities we create by designing for the commercial after-life next time you are texting a friend, emailing a colleague, or driving a car (not at the same time!)
Further reading and references:i McDonough, W., Baungarten, M. Cradle to Cradle. North Point Press, New York, 2002
ii Petretec - Dupont's Technology for Polyester Regeneration http://greenchem.uoregon.edu/Pages/Overview.php?ID=60
iii Jimenez-Gonzalez and Constable, Green Chemistry and Engineering – A practical design approach. John Wiley and Sons, 2010
-
Material and energy inputs should be renewable rather than depleting
Contributed by Dr. David Shonnard, Robbins Chair and Professor, Department of Chemical Engineering, Director of the Sustainable Futures Institute, Michigan Technological University, Houghton, MI USA
The Earth contains finite resources to support sustainable development into the future (unless mankind invents methods to import enormous quantities of raw materials from off the planet-not likely in the near term). As the human population on Earth increases to a projected peak of 9-10 billion from its current level of 7 billion by 2100 and as standards of living rise for many in developing countries, ever increasing pressure will be exerted on finite resources. As increased demand meets an ever-shrinking resource supply, prices for commodity materials and energy will rise. Evidence of this is already happening for petroleum whose current price of approximately $100/barrel is roughly 2.5 times higher than the average from 1987-2000 of $40/barrel (expressed in constant U.S. dollars). There are environmental costs as well to continued reliance on non-renewable raw materials, for example environmental degradation like open pit mines, waste piles, and climate warming by release of CO2 from combustion of fossil fuels (petroleum fuels, natural gas, and coal).
Renewable materials and energy can help address some of these negative effects. As noted in the discussion of Green Chemistry Principle #7 by Dr. Richard Wool, the photosynthetic production of biomass from the Earth’s land base is many more times the required amount to provide for all human material and energy needs through a bio-based economy. To utilize these renewable raw materials, innovations in Green Chemistry and Engineering are needed. Research in academia, government, and the private sector is making progress toward commercial production of advanced biofuels and other products, as the following examples show. Pyrolysis and catalytic hydrotreatment can produce hydrocarbon fuels from biomass or municipal solid waste in a matter of seconds rather than millions of years required by nature to accomplish the same result. The use of wood in buildings leads to removal of CO2 from the atmosphere and storage of carbon in stable forms for many decades, thereby helping mitigate effects of climate warming. High production volume chemicals such as ethylene are being produced commercially using sugarcane ethanol as a raw material. Regenerated cellulose fabric is being obtained from hardwood raw materials using an organic solvent spinning process for use in clothing and other fabrics. Solar, wind, hydro, and geothermal electricity sources are low emitters of greenhouse gases (GHGs) and their use in chemical manufacturing can lower the GHG intensity of the chemical industry.
While research is leading to innovative new processes and products based on renewable feedstocks, chemicals and fuels obtained from them may not always be more sustainable than identical products derived from fossil resources, and therefore caution must be used when renewable feedstocks are employed. For example, indigo extracted from plant materials is much more energy intensive than when obtained through a synthetic organic chemistry route (Shonnard et al. 2003). Biofuels derived from energy crops grown on agricultural lands that may displace food production have the potential (if not properly managed) to emit more CO2than the savings relative to petroleum fuels (Searchinger et al., 2008). These lessons demonstrate that prior to introducing chemicals, plastics, or other products derived from renewable feedstocks into the market on a large scale a comprehensive “cradle-to-grave” environmental sustainability assessment should be conducted. This will assure that the new products have advantages environmentally and socio-economically compared to conventional products.
Searchinger T, Heimlich R, Houghton RA, Dong F, Elobeid A, Fabiosa J, Tokgoz S, Hayes D, Yu TH (2008) Use of U.S. croplands for biofuels increases greenhouse gases through emissions from land-use change. Science. DOI: 10.1126/science.1151861; 10.1126/science.1151861
Shonnard, D.R., Kicherer, A., and Saling, P., 2003, Industrial Applications Using BASF Eco-Efficiency Analysis: Perspectives on Green Engineering Principles, Environmental Science and Technology, 37(23), 5340-5348.
* Anastas, P.T., and Zimmerman, J.B., "Design through the Twelve Principles of Green Engineering", Env. Sci. and Tech., 37, 5, 94A-101A, 2003.


