What molecule am I?
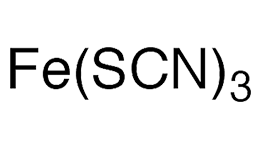

Iron(III) thiocyanate [Fe(SCN)3], aka ferric thiocyanate, is an iron salt with a striking deep red—almost black—color as a solid and a blood-red color in aqueous solution (see image). It first appeared in the chemical literature in 1884 in an article in the long-defunct Dinglers Polytechnisches Journal. The article, by German chemists L. Liechti and W. Suida was abstracted in the Journal of the American Chemical Society the same year; it described the properties of iron and aluminum thiocyanates (then called sulfocyanates) and their use in dyeing.
Fe(SCN)3’s characteristic color makes it useful for determining the existence and quantification of iron(III) in aqueous solution. When a colorless thiocyanate salt such as sodium thiocyanate1 (NaSCN) is added to the test solution, the bright red color appears, and its concentration can be quantified colorimetrically.
Fe(SCN)3 has additional uses in analytical chemistry:
- In 1984, Seiji Murai at the Industrial Research Institute of the Kanagawa Prefecture (Yokohama) reported that polyoxyethylene-based nonionic surfactants formed complexes with Fe(SCN)3. After extraction into 1,2-dichloroethane, the polymer concentration could be measured spectrophotometrically at 510 nm. A similar method was reported by Eiko Nakamura and Aki Futatsugi at Yokohama National University in 2000.
- Fe(SCN)3 frequently has been used to measure iodine concentrations in various substances. In a 1995 example, Youlin Chen at Suzhou School of Commerce (China) used a redox system consisting of arsenous acid (H3AsO3), cerium(IV) sulfate [Ce(SO4)2], and an iron(II) salt to determine trace iodine in table salt. The net oxidation reaction forms iron(III), which, upon addition of excess thiocyanate, forms the red Fe(SCN)3, again measured by spectrophotometry.
- Similar methods are used to determine peroxides. In 2019, Temel kan Bakir at Kastamonu University (Turkey) measured the peroxidation kinetics of palm and nut oils by adding iron(II) to oil emulsions and determined the amount of peroxide-generated iron(III) by adding thiocyanate and measuring Fe(SCN)3 spectrophotometrically.
1. CAS Reg. No. 540-72-7.
Iron(III) thiocyanate hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Acute toxicity, dermal, category 4 | H312—Harmful if in contact with skin | |
Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
Acute toxicity, inhalation, category 4 | H332—Harmful if inhaled | |
| Short-term (acute) aquatic hazard, category 3 | H402—Harmful to aquatic life | |
| Long-term (chronic) aquatic hazard, category 3 | H412—Harmful to aquatic life with long lasting effects | |
*Hazard information for iron(III) thiocyanate is not available. This information is for chemically similar sodium thiocyanate.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecule in the News
Streptomycin1 was the Molecule of the Week for October 21, 2014. It is an aminoglycoside drug that was introduced in the 1940s for treating tuberculosis and plague. Later, it became used as a pesticide to control bacterial diseases in fruit, vegetable, and ornamental crops.
In 2021, the US Environmental Protection Agency approved the use of spraying streptomycin on citrus crops to control infestations such as citrus greening disease. Shortly thereafter, environmental groups sued EPA for two alleged failures: to assess risks to pollinators and to consider whether streptomycin use increases risks of antibiotic resistance. Last month, a federal court reversed the approval and required EPA to evaluate the risks cited by the plaintiffs.
1. CAS Reg. No. 57-92-1.
Molecule in the News
MOTW highlights molecules that appear from major news outlets. See this week's edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Iron(III) thiocyanate
fast facts
| CAS Reg. No. | 4119-52-2 |
| SciFinder nomenclature | Thiocyanic acid, iron(3+) salt |
| Empirical formula | C3FeN3S3 |
| Molar mass | 230.09 g/mol |
| Appearance | Deep red hygroscopic crystals or powder |
| Melting point | Decomposes |
| Water solubility | Very soluble |
MOTW update
Adenosine triphosphate1 (ATP) was the Molecule of the Week for March 8, 2021. It is one of the most important biomolecules because it works with the enzyme ATP triphosphatase to transfer energy to the cells of all life forms.
ATP was discovered in 1929, and 95 years later scientists are still learning about it. This month, Divya Kota, Ramesh Prasad, and Huan-Xiang Zhou* at the University of Illinois Chicago reported that ATP mediates phase separation of basic intrinsically disordered proteins by bridging intermolecular interaction networks. The authors state that highly concentrated ATP droplets “have some of the lowest interfacial tension (≈25 pN/μm) but high zero-shear viscosities (1–15 Pa∙s) due to the bridged protein networks, and yet their fusion has some of the highest speeds (≈1 μm/ms).”
1. CAS Reg. No. 56-65-5.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

