What molecule am I?
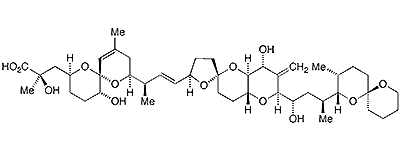
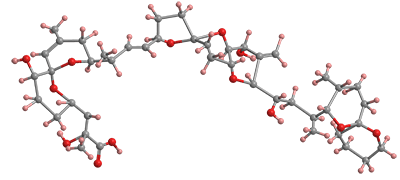
Okadaic acid1, a nasty marine toxin, is a complex multiring polyether found in certain dinoflagellates, single-cell eukaryotes of the genera Dinophysis and Thorecta. It typically accumulates in sea sponges and shellfish. It was first discovered and characterized in 1981 by Paul J. Scheuer, Jon Clardy, Francis J. Schmitz, and collaborators at the University of Hawaii at Manoa (Honolulu), Fujisawa Pharmaceutical (Tokyo), Cornell University (Ithaca, NY), and the University of Oklahoma (Norman) in the sponges Halichondria okadai (for which it was named) and H. melandocia.
Okadaic acid is a potent inhibitor of serine–threonine phosphatase subclasses PP2A, PP1, and PP2B. (PP stands for protein phosphatase.) It is the inhibition of this enzyme that is a primary cause of diarrheic shellfish poisoning (DSP) in humans. In addition to diarrhea, DSP can cause abdominal pain, nausea, and vomiting.
In 2013, Vanessa Valdiglesias and coauthors at the University of A Coruña (Spain) published a review of other harmful effects of okadaic acid. They discussed studies that reported the compound’s role in cytotoxicity, genotoxicity, immunotoxicity, embryotoxicity, neurotoxicity, and carcinogenicity. At that time, no reported data indicated that these toxicities cause chronic or subchronic effects in humans; but the authors believed that more research should be done in this area. On the positive side, they stated that okadaic acid is a useful tool for studying cellular signaling because of its selective inhibition of protein phosphatase activity.
For updated information on okadaic acid, see the molecule’s ScienceDirect page.
1. SciFinder: 1,7-dioxaspiro[5.5]undec-10-ene-2-propanoic acid, α,5-dihydroxy-α,10-dimethyl-8-[(1R,2E)-1-methyl-3-[(2R,4′aR,5R,6′S,8′R,8′aS)-octahydro-8′-hydroxy-6′-[(1S,3S)-1-hydroxy-3-[(2S,3R,6S)-3-methyl-1,7-dioxaspiro[5.5]undec-2-yl]butyl]-7′-methylenespiro[furan-2(3H),2′ (3′H)-pyrano[3,2-b]pyran]-5-yl]-2-propen-1-yl]-, (αR,2S,5R,6R,8S)-.
Okadaic acid hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 3 | H301—Toxic if swallowed | |
| Acute toxicity, dermal, category 3 | H311—Toxic in contact with skin | |
Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
Acute toxicity, inhalation, category 3 | H331—Toxic if inhaled | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules from the journals
Titanium diboride1 (TiB2) is one of the hardest known ceramic materials, especially in the temperature range 1000–3000 °C. It has other excellent properties, including high thermal and electrical conductivities and the highest elastic modulus, fracture toughness, compressive strength, and melting point of all boride ceramics.
Last month, Satadru Chakrabarty and Kabeer Jasuja* at the Indian Institute of Technology Gandhinagar described another valuable aspect of TiB2. When formed into a gel-like, oxygen-functionalized nanosheet dispersion, it reacts with ascorbic acid2 to change the color of the dispersion from yellow to orange. The reaction also disrupts the cross-links between the borate groups on the nanosheets that are responsible for the gel structure of the dispersion.
Pterodactylane3—tetracyclo[4.4.0.02,5.07,10]decane—is a hydrocarbon with four cyclobutane rings connected linearly. It was first synthesized in 1965 by Rangaswamy Srinivasan* and K. A. Hill at the IBM Watson Research Center (Yorktown Heights, NY) via the photolysis of benzene. Although the C4H10 product can exist in three positional isomers, only one isomer was produced.
Pterodactylane is so named because its structure resembles the wings of a pterodactyl in flight. It is also called [4]-ladderane, one of a series of fused cyclobutane molecules whose structures take the shape of a ladder.
In December, Stephen L. Craig, Todd J. Martínez, Yan Xia, and collaborators at Stanford University (CA), Duke University (Durham, NC), and Heinrich Heine University (Düsseldorf, Germany) described the mechanochemistry of pterodactylane. In one study, they prepared pterodactylanes with substituents on the central rung and on the end rung. When they applied outward forces to the substituents, they were surprised to find that the threshold force for bond breaking is dramatically lower for the central rung (0.7 nN) than the end rung (1.9 nN).
1. CAS Reg. No. 12045-63-5.
2. CAS Reg. No. 50-81-7.
3. CAS Reg. No. 4466-29-9.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Okadaic acid fast facts
| CAS Reg. No. | 78111-17-8 |
| Empirical formula | C44H68O13 |
| Molar mass | 805.00 g/mol |
| Appearance | White crystals or powder |
| Melting point | 164–166 °C 171–174 °Ca |
| Water solubility | Insoluble |
a. Both values appear in the chemical literature.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.